Health
Manitoba Man Faces $300K Treatment Denial for Rare Disease
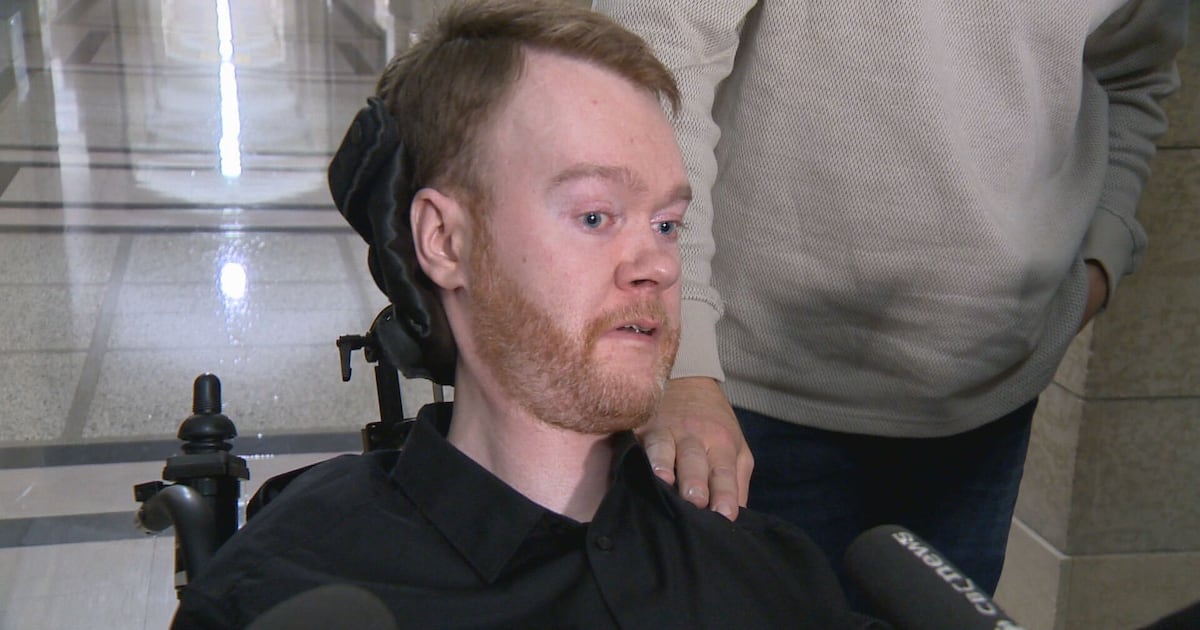
A Manitoban man with a rare genetic disease has been denied provincial coverage for a costly treatment, leaving him in a difficult position. Jeremy Bray, who suffers from spinal muscular atrophy (SMA), learned on March 25, 2024, that the medication he relies on, Risdiplam, will not be funded by the province, which could force him to pay more than $300,000 annually out of pocket.
Bray, 30, left the office of Health, Seniors, and Long-Term Care Minister Uzoma Asagwara visibly upset. “They were unwilling to commit to funding my treatment that runs out in a couple of weeks,” he said, expressing the emotional toll this decision has taken on him and his family. He described the situation as “tough,” highlighting the long struggle he has faced with his condition.
The treatment Bray uses is designed to manage SMA, a degenerative disease that progressively weakens muscles. Currently, Risdiplam is not approved by the Canadian Drug Agency (CDA) for adults over the age of 25, which has influenced the province’s decision. Minister Asagwara stated that the provincial government is adhering to the guidelines established by the CDA.
In a bid to assist Bray, the province successfully negotiated with the manufacturer, Roche, to extend his coverage under compassionate grounds back in May 2023. Asagwara emphasized the government’s commitment to advocating for Bray, stating, “As a government, we worked very, very hard fighting for Jeremy to ensure the CDA would perform an expedited review.”
Despite these efforts, Bray remains hopeful that the province might reconsider its stance on the treatment. “In April, it will be due to be assessed to see if the treatment is working,” he noted, expressing confidence in its effectiveness for his condition. Bray’s request is simple: he wants support from the province he has spent his entire life in.
Bray and his family are determined to continue the fight for coverage. They plan to explore all available options to secure funding for the treatment that is vital to his health. This situation has not only highlighted the challenges faced by individuals with rare diseases but has also sparked conversations about the accessibility of necessary treatments within Canada’s healthcare system.
As the review for Risdiplam approaches, all eyes will be on the CDA to see if it will take Bray’s case into account, potentially setting a precedent for others in similar circumstances. The outcome could have significant implications for those battling rare genetic conditions, underscoring the importance of timely access to life-changing medications.
-

 Science3 months ago
Science3 months agoToyoake City Proposes Daily Two-Hour Smartphone Use Limit
-

 Health3 months ago
Health3 months agoB.C. Review Reveals Urgent Need for Rare-Disease Drug Reforms
-

 Top Stories3 months ago
Top Stories3 months agoPedestrian Fatally Injured in Esquimalt Collision on August 14
-

 Technology3 months ago
Technology3 months agoDark Adventure Game “Bye Sweet Carole” Set for October Release
-

 World3 months ago
World3 months agoJimmy Lai’s Defense Challenges Charges Under National Security Law
-

 Lifestyle3 months ago
Lifestyle3 months agoVictoria’s Pop-Up Shop Shines Light on B.C.’s Wolf Cull
-

 Technology3 months ago
Technology3 months agoKonami Revives Iconic Metal Gear Solid Delta Ahead of Release
-

 Technology3 months ago
Technology3 months agoApple Expands Self-Service Repair Program to Canada
-

 Technology3 months ago
Technology3 months agoSnapmaker U1 Color 3D Printer Redefines Speed and Sustainability
-

 Technology3 months ago
Technology3 months agoAION Folding Knife: Redefining EDC Design with Premium Materials
-

 Technology3 months ago
Technology3 months agoSolve Today’s Wordle Challenge: Hints and Answer for August 19
-

 Business3 months ago
Business3 months agoGordon Murray Automotive Unveils S1 LM and Le Mans GTR at Monterey









