Science
Trump Extends Ethylene Oxide Compliance Deadline for Medical Devices
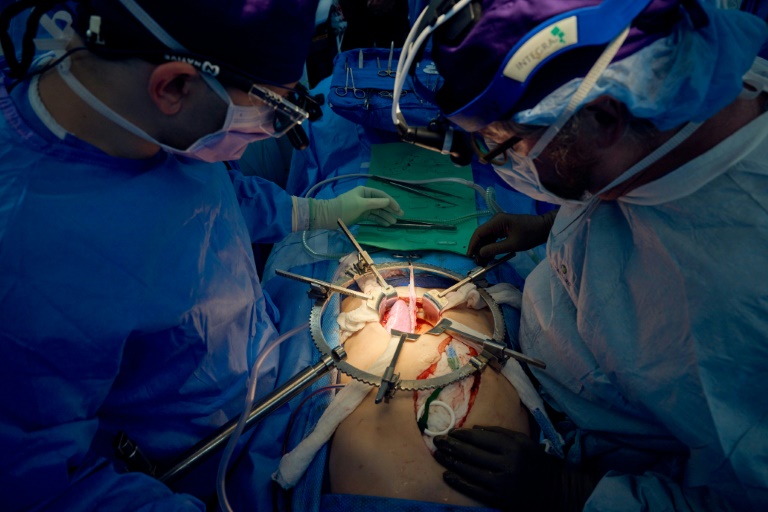
President Donald Trump has signed an executive order that grants a two-year extension for compliance with new regulations regarding the use of ethylene oxide (EtO) in medical device sterilization. This decision affects 39 specific facilities across the United States and reverses a previous policy aimed at reducing the reliance on this chemical, which has raised health and safety concerns.
Ethylene oxide is a crucial sterilant for many medical devices, although its use has been controversial due to its mutagenic properties. The U.S. Environmental Protection Agency (EPA) had issued a final rule in March 2024 that aimed to cut EtO emissions by over 90% through mandatory air pollution controls. This rule was designed to align with international standards, including ISO 10993-7 and ISO 11135, which set limits on residual levels of EtO in medical products.
The executive order extends the compliance deadline for these facilities, which will now have until the end of the two-year period to meet the new regulations. During this time, they will continue to adhere to the emissions and compliance obligations that were in place prior to the EPA’s revised EtO rule. This extension reflects concerns within the medical device industry over potential supply chain disruptions and logistical challenges associated with implementing the new requirements.
The order cites two key reasons for the extension: the lack of commercially viable technology to comply with the EtO regulations and a determination that the exemption is in the national security interests of the United States. The executive order invokes section 112(i)(4) of the Clean Air Act, which allows the President to grant compliance exemptions under specific circumstances.
Ethylene oxide is known to cause various health issues, including irritation of the eyes and respiratory system, headaches, and in severe cases, lung damage. The concern over its use is further amplified by its potential to induce mutations in DNA, which can lead to cancer. In Europe, the use of EtO has been declining, largely driven by stricter regulatory requirements.
In the U.S., approximately 50% of all sterile medical devices are treated with ethylene oxide, making it a vital component in the sterilization process for many products. Alternative methods, such as gamma radiation, are preferred by some regulators, including the UK’s medicines regulator, the MHRA, due to the risks associated with EtO residues.
The announcement of this extension has drawn mixed reactions from health experts and environmental advocates, who argue that it undermines public health efforts to mitigate the risks posed by hazardous substances. The decision is particularly noteworthy given the ongoing global push towards stricter environmental regulations and greater safety standards in the medical device industry.
As the compliance deadline approaches, stakeholders will be closely monitoring the implications of this executive order on both the health of the public and the future of medical device sterilization practices in the United States.
-

 Science2 months ago
Science2 months agoToyoake City Proposes Daily Two-Hour Smartphone Use Limit
-
 Health2 months ago
Health2 months agoB.C. Review Reveals Urgent Need for Rare-Disease Drug Reforms
-

 Top Stories2 months ago
Top Stories2 months agoPedestrian Fatally Injured in Esquimalt Collision on August 14
-

 Technology2 months ago
Technology2 months agoDark Adventure Game “Bye Sweet Carole” Set for October Release
-

 World2 months ago
World2 months agoJimmy Lai’s Defense Challenges Charges Under National Security Law
-

 Technology2 months ago
Technology2 months agoKonami Revives Iconic Metal Gear Solid Delta Ahead of Release
-

 Technology2 months ago
Technology2 months agoSnapmaker U1 Color 3D Printer Redefines Speed and Sustainability
-

 Technology2 months ago
Technology2 months agoAION Folding Knife: Redefining EDC Design with Premium Materials
-

 Technology2 months ago
Technology2 months agoSolve Today’s Wordle Challenge: Hints and Answer for August 19
-

 Business2 months ago
Business2 months agoGordon Murray Automotive Unveils S1 LM and Le Mans GTR at Monterey
-

 Lifestyle2 months ago
Lifestyle2 months agoVictoria’s Pop-Up Shop Shines Light on B.C.’s Wolf Cull
-

 Technology2 months ago
Technology2 months agoApple Expands Self-Service Repair Program to Canada