Science
Montreal’s AI-Powered Device Revolutionizes Cancer Surgery
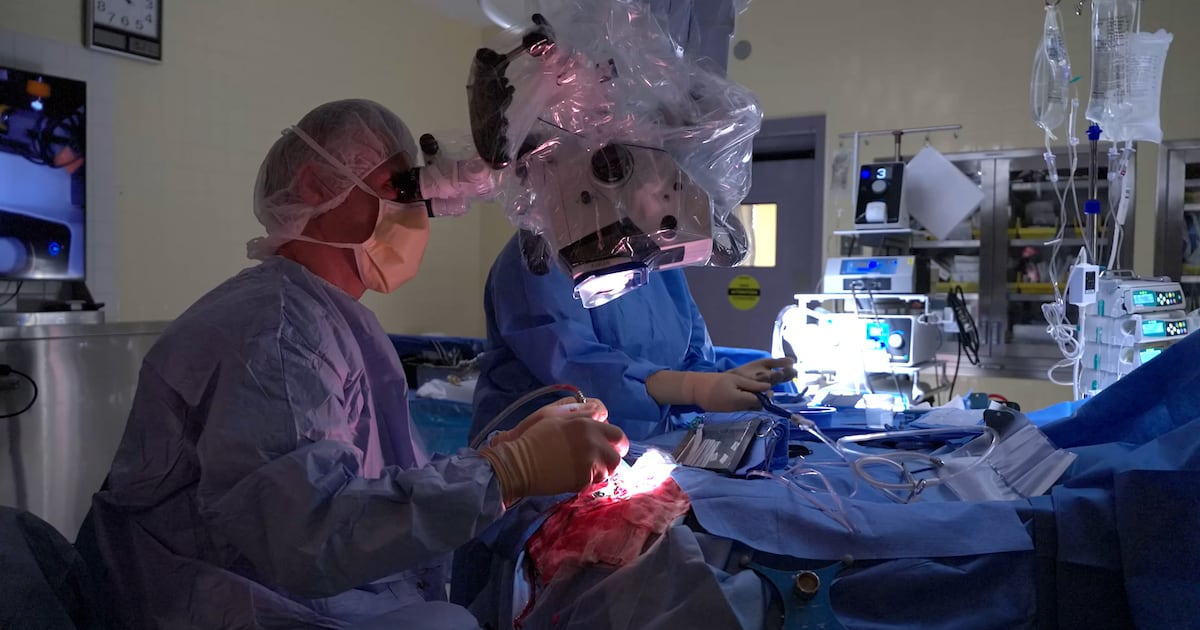
A groundbreaking surgical device developed in Montreal is significantly extending the lives of cancer patients, including one man who was given just 14 months to live. Peter Ross, diagnosed with a brain tumor nearly four years ago, credits this innovative technology for his unexpected recovery. Thanks to the SENTRY device, he and his wife Sandrine Menard are now preparing to welcome their first grandchild.
The SENTRY device utilizes artificial intelligence (AI) to accurately distinguish between cancerous and healthy tissue during surgery. According to Dr. Kevin Petrecca, a surgeon at Montreal Neuro and co-founder of SENTRY, the device achieves a remarkable accuracy rate: “98.7 per cent of the time, if it says it’s tumor, it’s tumor.” He further emphasizes that if SENTRY indicates normal brain tissue, it is correct 100 per cent of the time.
Transforming Surgical Outcomes
Cancer is often indiscernible to the naked eye or conventional technology, making surgeries challenging. SENTRY addresses this issue by providing immediate feedback to surgeons. “You make contact with the spot, and you will get feedback within less than three seconds if it contains tumor cells or not,” Dr. Petrecca explained. This capability enables surgeons to excise more cancerous tissue than previously possible.
The life-changing implications of SENTRY are profound. Dr. Petrecca asserts that using this device during surgery can extend a patient’s life from two to five times longer than anticipated. While SENTRY has primarily been tested for brain surgeries, its applications extend to detecting cancer in other areas, such as the breast and lungs.
Hundreds of patients have participated in clinical trials utilizing SENTRY, including Ross. “I take every extra day that’s given to me to go out and walk, and just enjoy life because I owe that to this surgery,” he shared. His experience underscores the potential of this technology to not only prolong life but also enhance the quality of life for survivors.
Future Prospects for SENTRY
The next significant milestone for SENTRY is obtaining approval from the U.S. Food and Drug Administration (FDA). A pivotal trial is scheduled for May 2024, and Ross is optimistic that the device will soon be accessible to a broader range of patients. He hopes that its approval will help others achieve the precious milestones he is now looking forward to in his life.
As advancements in medical technology continue to emerge, SENTRY stands out as a beacon of hope for cancer patients seeking more effective treatment options. The combination of AI and surgical innovation may well redefine cancer care and improve outcomes for countless individuals facing similar battles.
-

 Science2 months ago
Science2 months agoToyoake City Proposes Daily Two-Hour Smartphone Use Limit
-
 Health2 months ago
Health2 months agoB.C. Review Reveals Urgent Need for Rare-Disease Drug Reforms
-

 Top Stories2 months ago
Top Stories2 months agoPedestrian Fatally Injured in Esquimalt Collision on August 14
-

 Technology2 months ago
Technology2 months agoDark Adventure Game “Bye Sweet Carole” Set for October Release
-

 World2 months ago
World2 months agoJimmy Lai’s Defense Challenges Charges Under National Security Law
-

 Technology2 months ago
Technology2 months agoKonami Revives Iconic Metal Gear Solid Delta Ahead of Release
-

 Technology2 months ago
Technology2 months agoSnapmaker U1 Color 3D Printer Redefines Speed and Sustainability
-

 Technology2 months ago
Technology2 months agoAION Folding Knife: Redefining EDC Design with Premium Materials
-

 Technology2 months ago
Technology2 months agoSolve Today’s Wordle Challenge: Hints and Answer for August 19
-

 Business2 months ago
Business2 months agoGordon Murray Automotive Unveils S1 LM and Le Mans GTR at Monterey
-

 Lifestyle2 months ago
Lifestyle2 months agoVictoria’s Pop-Up Shop Shines Light on B.C.’s Wolf Cull
-

 Technology2 months ago
Technology2 months agoApple Expands Self-Service Repair Program to Canada