Health
B.C. Review Urges Reforms in Rare-Disease Drug Funding System
A leaked report from four years ago reveals that a review conducted by the British Columbia (B.C.) government recommended significant reforms to the province’s approach to funding and treating rare diseases with costly medications. These recommendations included calls for enhanced transparency, clearer communication, and stronger decision-making processes. This information comes as Premier David Eby recently reiterated the need for improvements in the system.
The 43-page report, obtained independently, highlights the urgency for change as spending on rare-disease drugs is projected to reach approximately $600 million annually by the end of the decade. Despite the urgency, many of the recommendations remain unimplemented. According to Dean Regier, an associate professor at the University of British Columbia’s School of Population and Public Health, the recommendations are still relevant today. He emphasized that navigating the challenges posed by expensive drugs for rare diseases is increasingly critical for health systems.
In a response, the B.C. Ministry of Health claimed that the “majority” of the report’s recommendations have either been implemented or are part of ongoing work. However, officials identified only two completed recommendations out of more than 300: the creation of a web page dedicated to expensive drugs for rare diseases and the establishment of an appeals process. The report itself underscores the need for much more than a website, calling for increased oversight, public engagement, and a comprehensive communication strategy.
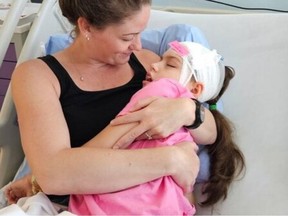
Eby and Health Minister Josie Osborne have both stressed the necessity for an overhaul of the current system, particularly following public backlash over the discontinuation of an $800,000 annual drug treatment for 10-year-old Charleigh Pollock, who suffers from an incurable degenerative disease. After the government reversed its decision last month, Eby acknowledged that the existing system was inadequate, leading to the resignation of ten members from an advisory committee.
The review, which was not made public until now, was delivered to the NDP government in the spring of 2021. Neither Eby nor Osborne referenced the past recommendations during their recent calls for reform. The Health Ministry indicated that staff are currently updating the list of recommendations to confirm their implementation status, with remaining suggestions to be assessed in light of the government’s ongoing review.
Dr. Sandra Sirrs, one of the advisory committee members who resigned, emphasized the importance of transparency in the management of expensive drugs for rare diseases. She noted that many recommendations from the 2021 report aimed at educating the public about these drugs have yet to be acted upon. Had these steps been taken earlier, the public may have been better prepared to understand the complexities surrounding the recent issues in B.C.
Regier, who participated in the original review, highlighted the ongoing need for public engagement in discussions about health funding, particularly regarding the allocation of resources for expensive treatments. He noted the necessity for a system to gather real-world data on these medications, as their effectiveness is not always clear. In the past year, B.C. spent $200 million on rare-disease drugs for approximately 600 patients.
The report’s detailed recommendations address not only the process for publicly funding rare disease drugs but also how to strengthen the approval system at both provincial and national levels. Some recommendations require cooperation from the federal government. B.C. Health Ministry officials pointed out that many issues can only be resolved at the federal level.
The review involved 17 participants, including representatives from the B.C. Ministry of Health, the Provincial Health Services Authority, and the Drug Benefit Council. The council, an independent body, provides evidence-based recommendations to the Ministry regarding drug listing for the pharmacare program. The report stresses the importance of transparency and effective communication, noting that addressing public concern over high drug costs will require strong leadership and a well-thought-out decision-making framework.
In light of the findings, the report advocates for establishing a supervisory body to oversee the implementation of the rare disease drug funding system, as well as implementing evidence standards and effectiveness thresholds for these costly treatments. Furthermore, the report suggests integrating rare-disease patients into the pharmacare program, a move that the Health Ministry has decided against.
The recommendations include a comprehensive communication and engagement strategy with an emphasis on transparency regarding decision criteria. It calls for a proactive approach to public relations to foster trust in government decisions and to ensure that patients and families feel heard.
In recent statements, Eby reiterated the need for reforms to enhance public understanding of the drug funding process. Osborne echoed this sentiment, stating that increased transparency could help build trust and ensure that patients are well-informed as decisions are made.
As the B.C. government grapples with the challenges posed by expensive drug treatments for rare diseases, the urgency for meaningful reform remains clear. The path forward will likely require a concerted effort from both provincial and federal authorities to address the complex issues that impact patients and their families across the province.

-

 Science3 months ago
Science3 months agoToyoake City Proposes Daily Two-Hour Smartphone Use Limit
-

 Top Stories3 months ago
Top Stories3 months agoPedestrian Fatally Injured in Esquimalt Collision on August 14
-

 Health3 months ago
Health3 months agoB.C. Review Reveals Urgent Need for Rare-Disease Drug Reforms
-

 Technology3 months ago
Technology3 months agoDark Adventure Game “Bye Sweet Carole” Set for October Release
-

 World3 months ago
World3 months agoJimmy Lai’s Defense Challenges Charges Under National Security Law
-

 Lifestyle3 months ago
Lifestyle3 months agoVictoria’s Pop-Up Shop Shines Light on B.C.’s Wolf Cull
-

 Technology3 months ago
Technology3 months agoKonami Revives Iconic Metal Gear Solid Delta Ahead of Release
-

 Technology3 months ago
Technology3 months agoApple Expands Self-Service Repair Program to Canada
-

 Technology3 months ago
Technology3 months agoSnapmaker U1 Color 3D Printer Redefines Speed and Sustainability
-

 Technology3 months ago
Technology3 months agoAION Folding Knife: Redefining EDC Design with Premium Materials
-

 Business3 months ago
Business3 months agoGordon Murray Automotive Unveils S1 LM and Le Mans GTR at Monterey
-

 Technology3 months ago
Technology3 months agoSolve Today’s Wordle Challenge: Hints and Answer for August 19








