Science
Montreal Surgical Device Uses AI to Transform Cancer Treatment
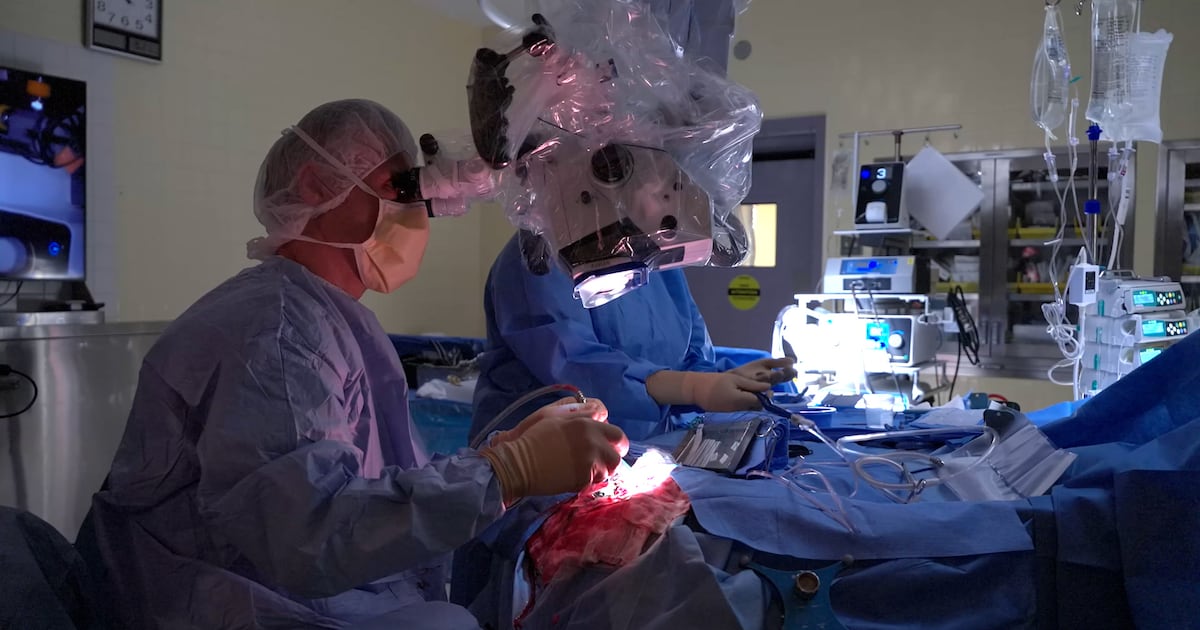
A groundbreaking surgical device developed in Montreal is transforming cancer treatment by utilizing artificial intelligence (AI) to extend patients’ lives. This innovative tool, known as SENTRY, has played a critical role in the recovery of patients like Peter Ross, who was given only 14 months to live following a brain tumor diagnosis nearly four years ago. Now, Ross and his wife, Sandrine Menard, are preparing for the arrival of their first grandchild, crediting their fortunate turn of events to this advanced technology.
Ross’s journey began when he was referred to Dr. Kevin Petrecca at Montreal Neuro. Petrecca, a neurosurgeon and co-founder of SENTRY, explained the device’s unique capabilities. SENTRY is a handheld tool that distinguishes between cancerous and healthy tissue during surgery. According to Petrecca, “98.7 per cent of the time, if it says it’s tumor, it’s tumor.” He further emphasized its reliability, stating that if SENTRY identifies tissue as normal, it is “normal brain 100 per cent of the time.”
Revolutionizing Surgical Precision
The precision of SENTRY allows surgeons to remove more cancerous tissue than was previously possible. Petrecca noted that the technology provides immediate feedback, indicating whether a tissue sample contains tumor cells within less than three seconds. “Cancer is not visible to the naked eye or any other technology,” he said, highlighting the device’s necessity in modern surgical practice.
Using SENTRY can significantly impact patient outcomes. Petrecca claims that the device can extend a patient’s life by two to five times longer than expected, depending on their specific condition. While the device has been primarily tested in brain surgery, its application extends to detecting cancer in other areas, such as the breasts and lungs. Hundreds of patients have participated in clinical trials to validate its effectiveness, including Ross, who shared, “I take every extra day that’s given to me to go out and walk and just enjoy life because I owe that to this surgery.”
Future Prospects and Regulatory Approval
The next major goal for SENTRY is to receive approval from the U.S. Food and Drug Administration (FDA). A pivotal trial is scheduled for May 2024, which will be crucial for the device’s future in the medical field. Ross expressed hope that the success of SENTRY will enable more patients to benefit from this life-saving technology and reach significant life milestones.
As the medical community continues to explore and expand the capabilities of AI in healthcare, the development of devices like SENTRY exemplifies a promising future for cancer treatment. With ongoing clinical trials and a focus on regulatory approval, the Montreal-made innovation is poised to change countless lives for the better.
-

 Science3 months ago
Science3 months agoToyoake City Proposes Daily Two-Hour Smartphone Use Limit
-

 Health4 months ago
Health4 months agoB.C. Review Reveals Urgent Need for Rare-Disease Drug Reforms
-

 Top Stories4 months ago
Top Stories4 months agoPedestrian Fatally Injured in Esquimalt Collision on August 14
-

 Technology3 months ago
Technology3 months agoDark Adventure Game “Bye Sweet Carole” Set for October Release
-

 World3 months ago
World3 months agoJimmy Lai’s Defense Challenges Charges Under National Security Law
-

 Lifestyle4 months ago
Lifestyle4 months agoVictoria’s Pop-Up Shop Shines Light on B.C.’s Wolf Cull
-

 Technology3 months ago
Technology3 months agoKonami Revives Iconic Metal Gear Solid Delta Ahead of Release
-

 Technology3 months ago
Technology3 months agoApple Expands Self-Service Repair Program to Canada
-

 Technology3 months ago
Technology3 months agoSnapmaker U1 Color 3D Printer Redefines Speed and Sustainability
-

 Technology3 months ago
Technology3 months agoAION Folding Knife: Redefining EDC Design with Premium Materials
-

 Technology4 months ago
Technology4 months agoSolve Today’s Wordle Challenge: Hints and Answer for August 19
-

 Business4 months ago
Business4 months agoGordon Murray Automotive Unveils S1 LM and Le Mans GTR at Monterey









